See feature articles below:
About Broad Street Alerts:
Big opportunities in Small Cap’s
Broad Street Alerts recent profiles and track record, 153% in verifiable potential gains for our members on the last 3 small cap alerts alone!
February 10th, 2016- (NASDAQ: BONT) opened $1.65/share hit a high of $3.00/share within 30 days our member potential gains- 83%
March 7th, 2016-(NYSE-MKT: FSI) opened at .91/share and hit 1.10/share within 5 days for gains of 21% for our members.
March 24th, 2016- (NASDAQ: ICLD) opened at $.77/share it a high of $1.15/share within 2 days for gains of 49% for our members.
These are numbers that make traders drool. Any trader in any market would fall all over themselves to see numbers like this. So if you’ve been on the fence, perhaps it’s time to start doing some research and verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest of the small-cap newsletters as the best in business.
We know with a large following comes a large responsibility as we have everyone from institutional investors to the beginner following our profiled securities in our newsletters. This is something we take very seriously always seeking small cap growth companies that have both near and long-term potential for our members.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP SMS/text alert service for free, simply text the word “Alerts” to the phone number 25827 from your cell phone.
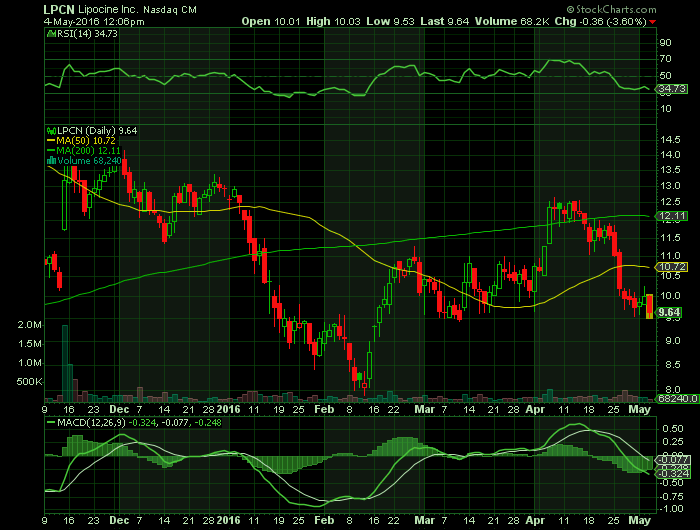
Report for: LPCN
SALT LAKE CITY, May 02, 2016 (GLOBE NEWSWIRE) — Lipocine Inc. (NASDAQ:LPCN), a specialty pharmaceutical company, today announced that clinical data for lead candidate TLANDO™ (“LPCN 1021”) will be presented in poster and podium presentations at the 2016 American Urological Association Annual Meeting being held May 6-10 in San Diego, CA. TLANDO is an oral testosterone replacement therapy (“TRT”) product candidate being developed for adult males with conditions associated with a deficiency or absence of endogenous testosterone, also known as hypogonadism.
Details are as follows:
Title: Hypogonadal Men with Sexual Function Disorder Benefit from LPCN 1021 (Oral Testosterone) – SOAR (Study of Androgen Replacement) Trial
Abstract No.: MP76-10
Date/Time: Monday, May 9, 2016, 3:30 – 5:30 p.m. PT
Location: San Diego Convention Center, Room 24
Presenter: Culley C. Carson III, MD
Rhodes Distinguished Professor, University of North Carolina Department of Urology
President, American Society for Men’s Health
Congress President, International Society of Men’s Health
Title: Long-term Safety and Tolerability of Oral Testosterone (LPCN 1021) in Hypogonadal Men: Results from the 52-Week Phase 3 Study
Abstract No.: PD50-06
Date/Time: Tuesday, May 10, 2016, 10:30 a.m. – 12:30 p.m. PT
Location: San Diego Convention Center, Room 23AB
Presenter: Mohit Khera, MD
Associate Professor, Urology, Baylor College of Medicine
Director, Laboratory for Andrology Research
Medical Director, Houston Hospital for Specialized Surgery
About TLANDO
TLANDO is a novel twice-a-day oral testosterone replacement therapy product candidate that is designed to help restore normal testosterone levels in hypogonadal men. The safety and efficacy of TLANDO is currently under FDA review. Lipocine expects TLANDO will help fulfill an unmet need in the treatment of hypogonadism. The current testosterone market primarily uses short-acting injectable products as well as topical products that carry an FDA “black box” warning related to inadvertent transfer of testosterone to others. According to the IMS Health database, an average of half a million prescriptions a month have been dispensed in 2016 for TRT.
About Lipocine
Lipocine Inc. is a specialty pharmaceutical company developing innovative pharmaceutical products for use in men’s and women’s health using its proprietary drug delivery technologies. TLANDO, an oral testosterone replacement therapy product candidate, demonstrated positive efficacy and safety results in Phase 3 testing and has a New Drug Application under review with the FDA. LPCN 1111, a next-generation oral testosterone replacement therapy product with once-daily dosing, is currently in Phase 2 testing. LPCN 1107, which has the potential to become the first oral hydroxyprogesterone caproate product indicated for the prevention of recurrent preterm birth, is currently in Phase 1 testing and has been granted orphan drug designation by the FDA. For more information, please visit www.lipocine.com
Source – Company Press Release
Broad street alerts has not been compensated for the mention of any publicly traded companies in this article nor do we own positions in any of the companies in this article.
Stock market
Hot small cap stocks
small cap stock picks
Biotech stocks
FDA approval stocks
FDA calendar
Trade stocks
Become a day trader
Day trade stocks for a living
PDUFA date set
micro cap stocks
Best stocks 2016
Hottest small cap stocks
Best stock picks
Who to follow for stock picks
Apple news stock picks
Stock picks on apple news