See feature articles below:
About Broad Street Alerts:
Big opportunities in Small Cap’s
Broad Street Alerts recent profiles and track record, 217% in verifiable potential gains for our members on the last 4 small cap alerts alone!
February 10th, 2016- (NASDAQ: BONT) opened $1.65/share hit a high of $3.00/share within 30 days our member potential gains- 83%
March 7th, 2016-(NYSE-MKT: FSI) opened at .91/share and hit 1.10/share within 5 days for gains of 21% for our members.
March 24th, 2016- (NASDAQ: ICLD) opened at $.77/share it a high of $1.15/share within 2 days for gains of 49% for our members.
April 11th, 2016 – (NASDAQ: FNJN) called at $1.07/share hit $1.76/share in 3 days for 64% gains for our members.
These are numbers that make traders drool. Any trader in any market would fall all over themselves to see numbers like this. So if you’ve been on the fence, perhaps it’s time to start doing some research and verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest of the small-cap newsletters as the best in business.
We know with a large following comes a large responsibility as we have everyone from institutional investors to the beginner following our profiled securities in our newsletters. This is something we take very seriously always seeking small cap growth companies that have both near and long-term potential for our members.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP SMS/text alert service for free, simply text the word “Alerts” to the phone number 25827 from your cell phone.
Oasmia Pharma (OASM) Announces Positive Paclical/Apealea Phase 3 Data; non-Inferiority Noted
Oasmia Pharmaceutical AB (Nasdaq: OASM) announced positive overall survival results for Paclical/Apealea in the Phase III study that included a total of 789 patients with epithelial ovarian cancer. These preliminary results showed non-inferiority between the two treatment groups of Paclical/Apealea in combination with carboplatin versus Taxol in combination with carboplatin. In fact, the overall survival in patients completing 6 treatment cycles was 25.7 months in patients that had received the Paclical/Apealea combination compared to 24.8 months in patients that had received the Taxol combination.
The results from the evaluation of the OS data confirm previous findings from June 2014 that the study had met the primary endpoint of Progression Free Survival (PFS) favoring Paclical/Apealea, and strengthens the positive risk/benefit profile for Paclical/Apealea published in October 2014. Earlier this year, Oasmia applied for marketing approval of Apealea (the alternatively branded name for Paclical) in the European Union for treatment of ovarian cancer. This overall survival data will be added to the EMA application and will form the basis of the marketing application to the FDA in the United States.
“It was expected that the analysis of the OS data would show non-inferiority and confirmation of the PFS results, two key factors for why we believe Apealea is an alternative to the presently available treatments of ovarian cancer,” said Margareta Eriksson, Vice President of Clinical Development at Oasmia Pharmaceutical. “Ovarian cancer is a fatal disease, one that is the fifth leading cause of cancer related deaths in women, and of which it is estimated that there will be over 22,000 new cases in the United States in 2016. Today, the treatment is designed to postpone fatality and to improve the quality of life for these patients.”
“These results are very important for the further development of Oasmia’s product pipeline, as the new data will add value to Apealea’s application for marketing approval in the EU and facilitate the marketing approval process in the United States,” said Julian Aleksov, Executive Chairman of Oasmia Pharmaceutical. “With recent reports forecasting the global market for drugs treating ovarian cancer will reach $1.71 billion in 2019, there exists a largely unmet need for novel therapies in oncology. We believe that Paclical/Apealea has tremendous potential to take significant market share in all major markets as we continue to commercialize and distribute the product.”
The overall survival data is requirement for a marketing authorization based on non-inferiority in the USA and Oasmia plans to submit an application to the FDA for approval of Apealea for treatment of ovarian cancer in the end of 2016/2017.
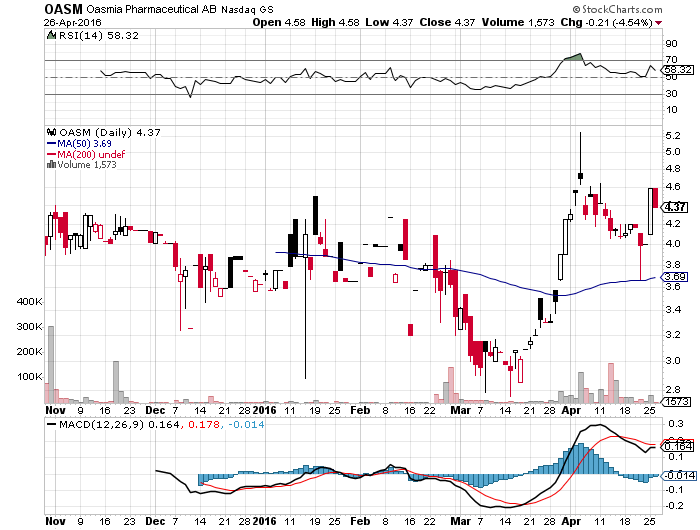
Source- Street Insider
Broad street alerts has not been compensated for the mention of any publicly traded companies in this article nor do we own positions in any of the companies in this article.
Broad Street Alerts was previously compensated eighteen thousand five hundred dollars by star media llc for the mention of FNJN however, that contract has expired.