See feature articles below:
About Broad Street Alerts:
Big opportunities in Small Cap’s
Broad Street Alerts recent profiles and track record, 153% in verifiable potential gains for our members on the last 3 small cap alerts alone!
February 10th, 2016- (NASDAQ: BONT) opened $1.65/share hit a high of $3.00/share within 30 days our member potential gains- 83%
March 7th, 2016-(NYSE-MKT: FSI) opened at .91/share and hit 1.10/share within 5 days for gains of 21% for our members.
March 24th, 2016- (NASDAQ: ICLD) opened at $.77/share it a high of $1.15/share within 2 days for gains of 49% for our members.
These are numbers that make traders drool. Any trader in any market would fall all over themselves to see numbers like this. So if you’ve been on the fence, perhaps it’s time to start doing some research and verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest of the small-cap newsletters as the best in business.
We know with a large following comes a large responsibility as we have everyone from institutional investors to the beginner following our profiled securities in our newsletters. This is something we take very seriously always seeking small cap growth companies that have both near and long-term potential for our members.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP SMS/text alert service for free, simply text the word “Alerts” to the phone number 25827 from your cell phone.
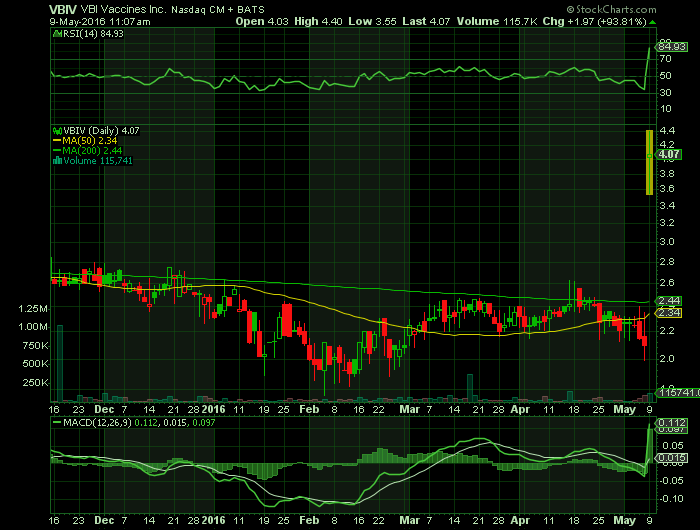
Article for: VBIV
CAMBRIDGE, Mass., May 09, 2016 (GLOBE NEWSWIRE) — VBI Vaccines Inc., a Delaware corporation (“VBI”), and SciVac Therapeutics Inc., a British Columbia corporation (“SciVac”), are pleased to announce the completion of their previously announced merger transaction, whereby SciVac acquired VBI. VBI survives the merger as a wholly owned subsidiary of SciVac.
Upon completion of the merger, SciVac changed its name to VBI Vaccines Inc. and will commence trading on The NASDAQ Capital Market under the symbol “VBIV” at market open on March 9, 2016.
The merger creates a commercial stage company with an approved hepatitis B vaccine, a pipeline of preventative and therapeutic vaccine candidates, and two novel technology platforms.
Jeff Baxter, President and Chief Executive Officer of VBI, Dr. David Anderson, Chief Scientific Officer of VBI, and Jim Martin, SciVac’s Chief Financial Officer, will continue in those same officer roles with the combined company. Dr. Curtis Lockshin, SciVac’s former Chief Executive Officer, will assume the role of Chief Technology Officer of the combined company. Dr. Steven Gillis, Chairman of the Board of VBI, will serve as Chairman of the Board of the combined company, and will be joined on the board by Adam Logal and Steven Rubin, both of OPKO Health, Inc. (OPK), the combined company’s largest shareholder.
“We are thrilled and excited to announce the completion of this merger, which we believe dramatically propels the future development of the assets within each legacy company,” said Mr. Baxter. “SciVac brings Sci-B-Vac, a licensed and marketed third-generation hepatitis B vaccine, which complements a highly innovative infectious disease pipeline of product candidates, the lead program of which targets cytomegalovirus. We feel incredibly fortunate to be able to contribute to the growth of Sci-B-Vac, a pioneering product that is approved in smaller markets, but we believe is capable of being scaled and developed in late-stage clinical trials in order to seek additional approvals in Europe, the United States, Japan, and other large markets.”
Following the merger, the combined company believes it will be well-positioned to advance its infectious disease and immuno-oncology vaccine candidates, as well as its proprietary technology platforms. Current development programs include:
Infectious Disease
Sci-B-Vac is a commercial stage hepatitis B (“HBV”) vaccine that mimics all three viral surface antigens of the hepatitis B virus and is free of any next-generation adjuvant. Sci-B-Vac offers rapid onset of protection, high levels of anti-HBV antibodies, and can be administered at lower doses than competing HBV vaccines. Sci-B-Vac is approved in Israel and in 14 other countries and has demonstrated a favorable safety and efficacy profile in over 300,000 patients.
VBI is developing a vaccine to prevent cytomegalovirus (“CMV”) infection. CMV is a leading cause of serious birth defects in newborns when a mother is infected during pregnancy. Based on preclinical data, VBI has completed GMP manufacturing of its lead candidate for use in Phase I trials; VBI expects to evaluate safety, tolerability, and also immunological proof of concept in humans during Phase I trials.
VBI is developing a vaccine to prevent respiratory syncytial virus (“RSV”) infection. RSV is a respiratory virus that infects the lungs and airways. VBI has been awarded grant funding by the National Research Council-Industrial Research Assistance Program (“NRC-IRAP”) to develop a vaccine candidate that expresses the pre-fusion RSV-F protein.
Immuno-Oncology
More
VBI is developing a therapeutic vaccine candidate for glioblastoma multiforme (“GBM”). GBM is among the most common and aggressive malignant primary brain tumors in humans. With its novel approach, VBI intends to create a GBM immunotherapy that will stimulate the patient’s own immune system to identify and kill GBM cancer cells, with the goal of creating a commercially-viable therapy that is more effective and tolerable than current treatments.
VBI is developing additional undisclosed therapeutic vaccine candidates that utilize the eVLP Platform to deliver foreign viral antigens that are highly associated with multiple solid tumors.
Technology Platforms
VBI’s eVLP Platform allows for the design of enveloped (“e”) virus-like particle (“VLP”) vaccines. eVLPs are an innovative new class of synthetic vaccines that are designed to closely mimic the structure of viruses. The eVLP Platform has given rise to VBI’s CMV, RSV, and GBM vaccine candidates.
The LPV Platform is a proprietary formulation and process that allows vaccines and biologics to preserve stability, potency, and safety. VBI is currently leading broad research collaborations with GlaxoSmithKline Biologics SA and Sanofi Pasteur to evaluate the LPV Platform.
In meetings held on January 29, 2016 and May 5, 2016, respectively, shareholders collectively holding approximately 55% of the issued and outstanding SciVac common shares and stockholders collectively holding approximately 75% of the issued and outstanding VBI common stock voted in favor of the merger.
Broad street alerts has not been compensated for the mention of any publicly traded companies in this article nor do we own positions in any of the companies in this article.
Stock market
Hot small cap stocks
small cap stock picks
Biotech stocks
FDA approval stocks
FDA calendar
Trade stocks
Become a day trader
Day trade stocks for a living
PDUFA date set
micro cap stocks
Best stocks 2016
Hottest small cap stocks
Best stock picks
Who to follow for stock picks
Apple news stock picks
Stock picks on apple news