See feature articles below:
About Broad Street Alerts:
Big opportunities in Small Cap’s
Broad Street Alerts recent profiles and track record, 153% in verifiable potential gains for our members on the last 3 small cap alerts alone!
February 10th, 2016- (NASDAQ: BONT) opened $1.65/share hit a high of $3.00/share within 30 days our member potential gains- 83%
March 7th, 2016-(NYSE-MKT: FSI) opened at .91/share and hit 1.10/share within 5 days for gains of 21% for our members.
March 24th, 2016- (NASDAQ: ICLD) opened at $.77/share it a high of $1.15/share within 2 days for gains of 49% for our members.
These are numbers that make traders drool. Any trader in any market would fall all over themselves to see numbers like this. So if you’ve been on the fence, perhaps it’s time to start doing some research and verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest of the small-cap newsletters as the best in business.
We know with a large following comes a large responsibility as we have everyone from institutional investors to the beginner following our profiled securities in our newsletters. This is something we take very seriously always seeking small cap growth companies that have both near and long-term potential for our members.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP SMS/text alert service for free, simply text the word “Alerts” to the phone number 25827 from your cell phone.
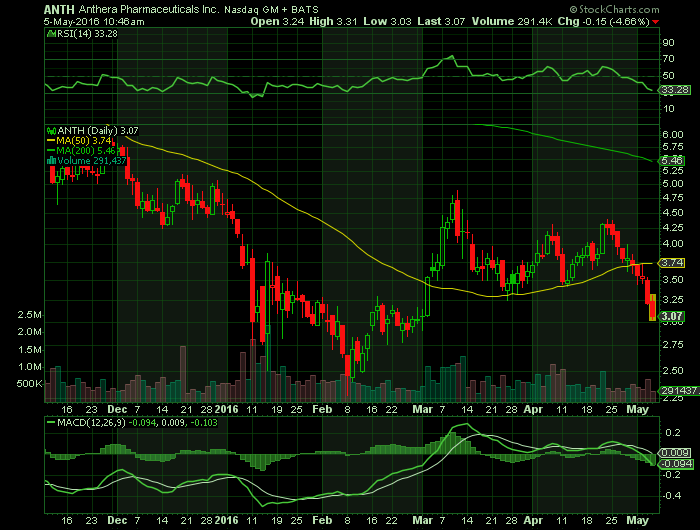
Report for: ANTH
HAYWARD, Calif., March 10, 2016 (GLOBE NEWSWIRE) — Anthera Pharmaceuticals, Inc. (Nasdaq: ANTH) today provided a business update and reported financial results for the fourth quarter and year ended December 31, 2015.
Recent Developments and Business Highlights:
Clinical Development
Sollpura™ (liprotamase)
SOLUTION Phase 3 Clinical Study to Expand into Europe
In September 2015, we began enrollment of the SOLUTION Phase 3 clinical study in the United States. The study will evaluate the efficacy and safety of Sollpura™ in capsule formulation in patients with cystic fibrosis who suffer from exocrine pancreatic insufficiency.
Patient accrual continues to meet our expectation with top line data targeted to be available at the end of 2016 or early 2017. For more information on the study, please visit https://clinicaltrials.gov/ct2/show/NCT02279498.
SIMPLICITY Pediatric Clinical Study expected to begin in Q2 2016
The SIMPLICITY clinical study will be the first study that uses Sollpura™ powder for oral solution. Sollpura™ will be supplied in a convenient, easy-to-administer sachet (packet, similar to sugar), which is dissolvable in about one tablespoon of water. After an initial cohort of older patients, the SIMPLICITY study will focus on administration of Sollpura™ to pediatric patients ranging in age from 28 days to seven years.
Blisibimod – Systemic Lupus Erythematosus (“SLE”)
Phase 3 CHABLIS-SC1 Clinical Study Enrollment Completed
We are on track to provide topline efficacy and safety data from the CHABLIS-SC1 study during Q3 2016.
Phase 3 CHABLIS 7.5 Clinical Study deployment continues on track
The CHABLIS 7.5 study will evaluate the efficacy and safety of blisibimod when administered on top of standard-of-care medication in patients with severe, seropositive SLE who are inadequately controlled with corticosteroids. Patient eligibility for this study is informed by responder traits identified in the Phase 2 study with blisibimod as well as the large Phase 3 programs with other BAFF inhibitors. For more information about the CHABLIS 7.5 study, please visit https://clinicaltrials.gov/ct2/show/NCT02514967.
Blisibimod – IgA Nephropathy
Phase 2 BRIGHT-SC Data Planned for Q2 2016
Following our receipt of a termination notice from Zenyaku and the subsequent expiration of the 120-day termination period on January 7, 2016, we elected to stop further enrollment in the BRIGHT-SC clinical study. We have enrolled 57 patients, 45 of which remained in the study. We plan to conduct a proof-of-concept efficacy analysis when substantially all patients have received a minimum of six months of therapy in Q2 2016. This analysis will examine the effects of blisibimod versus placebo in the proportion of qualifying patients who achieve a complete response (“CR”) and a partial response (“PR”) at six months. CR is defined as the proportion of patients with baseline UPE of greater than two grams per 24 hours, achieving UPE of less than one gram per 24 hours or a 50% reduction in UPE from baseline, and the proportion of patients with baseline UPE between one and two grams per 24 hours, achieving UPE of less than one gram per 24 hours and a 50% reduction from baseline. PR is defined as the proportion of patients achieving urinary protein excretion (“UPE”) of less than or equal to one gram per 24 hours. Based on our previous discussions with regulatory authorities, we believe an evaluation of the change in proteinuria could serve as a surrogate endpoint to support an accelerated or conditional approval in various key commercial geographies. Following the completion of the BRIGHT-SC study we intend to explore a Special Protocol Assessment from the US FDA to ensure final concurrence on the willingness to consider proteinuria as a surrogate endpoint, the timing for completion of enrollment of the post marketing confirmatory study and blisibimod’s eligibility to receive orphan designation.
Management Update
On January 7, 2016, we expanded our executive management team through the appointment of Craig Thompson as our President and Chief Operating Officer. In this role, Mr. Thompson will oversee both our late stage development programs and launch readiness efforts.
On March 7, 2016, our Board appointed Dr. James Pennington as our Interim Chief Medical Officer, effective April 1, 2016, following the departure of our current Chief Medical officer, Dr. Colin Hislop. Dr. Pennington joined Anthera in 2007 and has over 40 years of successful clinical development and commercialization experience including 14 drug approvals and 10 registration ex-US approvals including both small and large molecule therapeutics.
Summary of Financial Results
Cash Position. Cash and cash equivalents totaled $47 million as of December 31, 2015, compared to $2.6 million as of December 31, 2014. The increase in cash was mainly attributable to our March and July public offerings of common stock, sale of common stock through an at-the-market program, equity investment and cost reimbursement from Zenyaku, and a research grant from Cystic Fibrosis Foundation Therapeutics (“CFFT”), offset by cash used in operations.
Revenues. License and collaborative revenues for the fourth quarter and year ended December 31, 2015 totaled $1.9 million and $3.2 million, respectively. Following the receipt of a termination notice from Zenyaku to terminate a collaborative arrangement, we began to accelerate the amortization of our deferred revenue in the third quarter of 2015 to correspond with the shortened collaboration period and the amount was fully amortized by January 7, 2016. As a result we expect no significant revenues associated with the terminated Zenyaku collaboration in 2016.
R&D Expense. Research and development expenses for the fourth quarter and year ended December 31, 2015 totaled $8.6 million and $33.5 million, respectively, compared to $5.5 million and $21.8 million in the same periods in 2014. Research and development expenses increased in 2015 from 2014 primarily due to higher non-cash stock-based compensation recognized in 2015 as a result of option grants issued in 2015 and higher expense for manufacturing and clinical operations to support the ongoing CHABLIS-SC1 and BRIGHT-SC studies, as well as the initiation our Phase 3 SOLUTION study with Sollpura™. The increase was partially offset by $1.5 million in expense reimbursements from Zenyaku for certain development costs associated with blisibimod.
G&A Expense. General and administrative expenses for the fourth quarter and year ended December 31, 2015 totaled $1.9 million and $7.6 million, respectively, compared to $1.8 million and $6.6 million in the same periods in 2014. The increase in 2015 from 2014 is primarily due to higher non-cash stock-based compensation expense recognized in 2015 as a result of option grants issued in 2015 and higher expense related to professional services to support the growth of the Company.
Research Award. A Research award, granted to the Company in March of 2015 by CFFT and recorded as an offset to operating expense, totaled $1.2 million and $2.6 million for the fourth quarter and year ended December 31, 2015. The amount of research award we recognized represents the value prescribed to the milestones we achieved under the award agreement during 2015.
Net Loss. Net loss for the fourth quarter and year ended December 31, 2015 was $7.3 million, or $0.18 per basic and diluted share, and $35.2 million, or $0.99 per basic and diluted share, respectively, compared to $7.4 million, or $0.32 per basic and diluted share, and $29.6 million, or $1.36 per basic and diluted share, in the same periods in 2014. The increase in net loss was primarily related to the advancement of our blisibimod program and the initiation of Sollpura™ in a Phase 3 clinical study. The decrease in our loss per share both in the fourth quarter and fiscal year 2015 was attributable to increase in our common shares outstanding in 2015 due to two public offerings, takedown from our at-the-market offering and equity investment by Zenyaku.
About Anthera Pharmaceuticals
Anthera Pharmaceuticals is a clinical-stage biopharmaceutical company focused on developing and commercializing products to treat serious and life-threatening diseases, including exocrine pancreatic insufficiency due to cystic fibrosis, lupus, lupus with glomerulonephritis, and IgA nephropathy. Additional information on the Company can be found at www.anthera.com.
Source – Company Press Release
Broad street alerts has not been compensated for the mention of any publicly traded companies in this article nor do we own positions in any of the companies in this article.
Stock market
Hot small cap stocks
small cap stock picks
Biotech stocks
FDA approval stocks
FDA calendar
Trade stocks
Become a day trader
Day trade stocks for a living
PDUFA date set
micro cap stocks
Best stocks 2016
Hottest small cap stocks
Best stock picks
Who to follow for stock picks
Apple news stock picks
Stock picks on apple news