See feature articles below:
About Broad Street Alerts:
Big opportunities in Small Cap’s
Broad Street Alerts recent profiles and track record, 153% in verifiable potential gains for our members on the last 3 small cap alerts alone!
February 10th, 2016- (NASDAQ: BONT) opened $1.65/share hit a high of $3.00/share within 30 days our member potential gains- 83%
March 7th, 2016-(NYSE-MKT: FSI) opened at .91/share and hit 1.10/share within 5 days for gains of 21% for our members.
March 24th, 2016- (NASDAQ: ICLD) opened at $.77/share it a high of $1.15/share within 2 days for gains of 49% for our members.
These are numbers that make traders drool. Any trader in any market would fall all over themselves to see numbers like this. So if you’ve been on the fence, perhaps it’s time to start doing some research and verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest of the small-cap newsletters as the best in business.
We know with a large following comes a large responsibility as we have everyone from institutional investors to the beginner following our profiled securities in our newsletters. This is something we take very seriously always seeking small cap growth companies that have both near and long-term potential for our members.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP SMS/text alert service for free, simply text the word “Alerts” to the phone number 25827 from your cell phone.
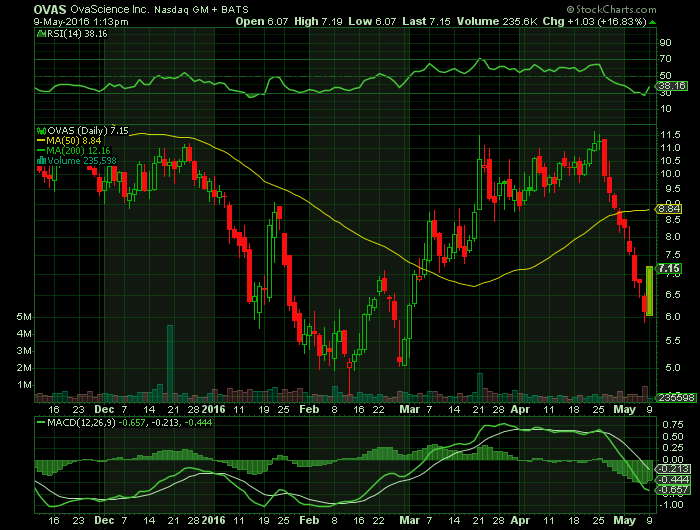
Article for: OVAS
Earnings Call Transcript
Herald Stock, OvaScience Inc. – CEO-Elect [3]
——————————————————————————–
Thank you, Rebecca, and good afternoon, everyone. I’m very pleased to be speaking with you today and I’m looking forward to taking over as CEO starting July 1. That said clearly, I am already engaged with the company and putting in place plans for our future success.
I wanted to take a moment to share with you my vision for OvaScience and our 2016 operational strategy before handing it over to Jeff and Michelle to review the first quarter financial results and our business highlights.
As I prepared to take over as CEO, I am inspired by the energy and commitments our employees bring to work every day, which is driven by our company’s mission — and mission to transform fertility by helping woman and couples build the families they deserve. I am also compelled by management’s ability to execute towards our goals.
In addition to the company’s enormous potential for future growth in the months and years ahead. In under five years, the talented team of scientists here at OvaScience has succeeded in creating and making augment a transformative fertility treatment globally available. And we’re also developing two additional potentially ground breaking options, OvaPrime and OvaTure.
As we look at the year ahead, we’re focused on three primary objectives or pillars that both serves as the foundation to grow our business. First, we aim to expand our operational platform and execute a target and expansion of the AUGMENT treatment with a narrow and deep focus on Canada and Japan as our primary commercial markets.
Second, we aim to increase our patient support services by building a patient centered operating model. This means on the ground teams in market to support patients and clinics. Third, we plan to continue to generate a robust data set to further validate our portfolio of novel fertility treatments and demonstrate their clinical benefit.
Regarding our first objective, we’re currently working to expand access to augment in key IVF markets by taking a narrow and deep approach to the commercial launch. We have already made significant progress in building our ongoing commercial operations and infrastructure particularly in Canada where in addition to existing partnerships; we recently entered into agreements with two new partner clinics who will begin offering the AUGMENT treatment commercially following the completion of a preceptorship training program.
From our experience in that market, we expect to draw meaningful conclusions to inform our strategy as we embark on comprehensive launches in other regions such as Japan. To this end, we will continue to enhance our patient services in part by shifting to a patient-centered marketing approach. With this type of commercial model, we plan to be more proactive in raising awareness and educating patients directly about augment.
In the case of fertility treatment, we believe patients are sophisticated consumers who are informed and will pursue breakthrough treatments.
Lastly, we’re committed to improving market confidence in our treatment paradigms by creating validating datasets to support the use of our treatments. The evidence for AUGMENT’s efficacy is already compelling. However, we look forward to data in the second half of 2017 from the ongoing egg allocation study conducted by our partner, the IVI Group, comparing standard IVF to Augment.
And with respect to the U.S., we are determining our FDA strategy and will update you on our progress by year-end. Beyond AUGMENT, we expected a debut on our path forward with OvaPrime by the end of this year.
We have an exciting year ahead with multiple catalysts and I’m confident in the company’s ability to execute and transition into our next phase of growth. I am very excited to work with everyone on the management team, our dedicated employees and our Board of Directors as we execute on our strategy. I look forward to keeping you updated on our progress.
And with that, I will turn the call over to Jeff.
——————————————————————————–
Jeffrey Young, OvaScience Inc. – CFO [4]
——————————————————————————–
Thank you, Herald, and good afternoon, everyone. Earlier this afternoon we issued a press release detailing our financial results for the first quarter of 2016. Now I’d like to take a few minutes to recap those results.
First quarter cash burned was $16.6 million and we ended the first quarter with cash, cash equivalents and short-term investments of $110.1 million. We currently believe this cash balance is sufficient to support our activities through key inflection points including our targeted expansion of AUGMENT in Canada and Japan, our ongoing study of AUGMENT in conjunction with the IVI Group in Spain, and a continued advancement of our leading fertility pipeline treatments OvaPrime and OvaTure.
Turning now to the financial results. Revenue for the first quarter was $146,000 compared to $15,000 in the same period last year. The revenue recognized in the first quarter relates 24 AUGMENT cycles at a blended price per treatment as compared to one AUGMENT cycle in the first quarter of 2015. In addition compared to the fourth quarter 2015, we increased the number of patients treated with AUGMENT from 14 to 24.
As we described in the fourth quarter call, we continue to anticipate an average or blended commercial price for the full year 2016 of approximately $7,000 per AUGMENT treatment cycle.
Net loss for the quarter was $21.8 million or $0.80 per share, as compared to the net loss of $17.2 million or $0.65 per share for the same period in 2015 and $20.6 million or $0.76 per share for the fourth quarter of 2015. We attribute this increase primarily to planned higher personnel and personnel related costs as well as costs associated with our international commercial expansion of AUGMENT.
Cost of revenue for the quarter was $1.2 million compared to $35,000 in the same period in 2015. This increase was primarily driven by the continued expansion of our commercial operations to support our current demand as well as our anticipated future growth.
Research and development expense for the quarter was $6 million compared to $5.7 million for the same period in 2015. This increase resulted primarily from a $1.1 million increase in employee compensation and employee related benefits, a $0.6 million increase in cost related to our clinical study being performed at the IVI Clinic in Spain, and a $0.5 million increase in facility and consulting cost. This was partially offset by a decrease of $2 million of stock-based compensation expense related to certain mark-to-market adjustments of our Founders’ shares that fully vested in the first quarter of 2015 and did not recur in 2016.
We expect research and development expense to increase as we continue to advance our program successfully towards commercialization and continue patient enrollment in our studies.
Selling, general and administrative expense for the three months ended March 31, 2016 was $14.5 million as compared to $11 million for the same period in 2015. This increase was primarily result of a $2.2 million increase in employee compensation and related benefits driven by the hiring of additional sales and general and administrative personnel to support our operations.
A $1.6 million increase in legal accounting tax and other related services, primarily to support our International growth and a $0.6 million increase in facility related costs. This was partially offset by a $1 million decrease of stock-based compensation expense related to the completion of the vesting of our Founders’ shares.
We expect selling, general and administrative expense to gradually increase as we continue to hire international sales and operational personnel to support our commercial efforts for the AUGMENT treatment and future treatments in our key regions.
In summary, the increase in spend in the first quarter of 2016 is aligned with our three primary objectives that as Harold mentioned will serve as a foundation to grow our business as we expand our operational platform to our focused regions, increase our patient support services through our in-market teams, and generate data to further validate our portfolio.
With that, I’ll turn the call over to Michelle. Michelle?
——————————————————————————–
Michelle Dipp, OvaScience Inc. – CEO and Executive Chair [5]
——————————————————————————–
Thank you, Jeff. I will now review recent developments. In March results supporting AUGMENT mechanism of action were published in a peer reviewed article in Scientific Reports, a Nature journal. This publication highlighted data from a study conducted by Dr. Justin St. John from the Hudson Institute of Medical Research, one of the world’s top medical research institutes. This study showed the importance of mitochondria in normal embryo development. Moreover, this study further supports the current body of evidence on the role of mitochondria from egg precursor cells in improving female fertility and improving pregnancy rates.
Beyond the scientific data, what’s most important is that we continue to see healthy babies being born. To-date, 30 babies have been born with the AUGMENT treatment and the first baby to be born with AUGMENT recently celebrated his first birthday.
As Herald noted, we are expanding our commercial presence in Canada. In the first quarter, we’ve signed two new agreements with clinics that will offer AUGMENT commercially. This includes one of the largest clinics in Canada.
Now turning to Japan, we completed enrollment in our non-commercial preceptorship training program at IVF Japan, where we are making progress towards commercialization. Internally, we continue to build our AUGMENT data set as we progress the enrollment of our ongoing egg allocation study with the IVI Group. As a reminder, this is a prospective controlled, double blind and randomized egg allocation study with an adaptive design to evaluate the success of standard IVF versus AUGMENT. We expect to report data in the second half of 2017.
Turning now to OvaPrime, we have also continued development efforts by advancing our clinical study. This study is designed to give clinicians hands on experience with the OvaPrime treatment and to evaluate OvaPrime’s safety and efficacy as measured by a woman’s hormone levels and follicular development. We plan to provide an update on the path forward for OvaPrime by year-end.
Finally, we’re continuing to advance our third product in development OvaTure, a potential next generation IVF treatment for women with compromised eggs, or those who are unwilling or unable to undergo hormone hyper-stimulation. We are currently maturing human egg precursor cells into human eggs and we plan to continue this work throughout 2016.
At OvaScience, we are shifting gears with an increased focus on commercial operations. We remain focused on our mission to bring novel fertility treatments to patients in need and we aim to become a global leader in fertility.
Source – Reuters
Broad street alerts has not been compensated for the mention of any publicly traded companies in this article nor do we own positions in any of the companies in this article.
Stock market
Hot small cap stocks
small cap stock picks
Biotech stocks
FDA approval stocks
FDA calendar
Trade stocks
Become a day trader
Day trade stocks for a living
PDUFA date set
micro cap stocks
Best stocks 2016
Hottest small cap stocks
Best stock picks
Who to follow for stock picks
Apple news stock picks
Stock picks on apple news