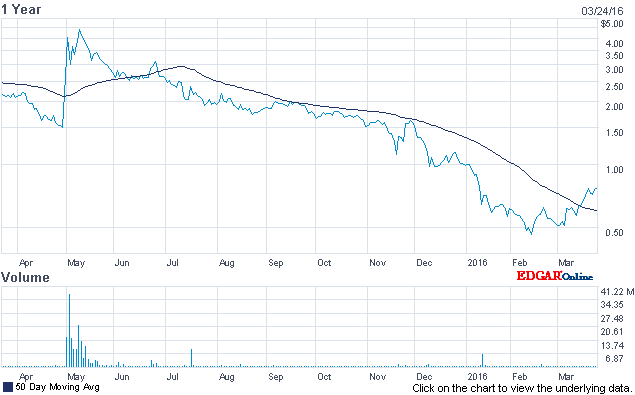
About Broad Street Alerts: — NVTA Release -Below
Big opportunities in Small Cap’s
Broadstreetalerts.com recent profiles and track record, 157% in verifiable potential gains for our
members in December 2015 alone.
December 29th, 2015- (NASDAQ: INVT) opened $1.35/share hit a high midday of $2.82/share over 100%
in gains for our members
December 15th, 2015-(NYSE-MKT: XXII) opened at $1.29 hit a high of $1.54 within 3 days for gains of
19% for our members.
December 2nd, 2015- (NASDAQ: TCCO) opened at $3.25 hit a high of $4.50 within 3 days for 38% gains
for our members.
***IN PLAY- Current profile sent at 3pm on March 24th, 2016 to our members up 17% so far (NASDAQ:
ICLD) full profile- https://broadstreetalerts.com/wp-content/uploads/2016/03/ICLD-profile.pdf
These are numbers that make traders drool. Any trader in any market would fall all over themselves to
see numbers like this. So if you’ve been on the fence, perhaps it’s time to start doing some research and
verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest
of the small-cap newsletters as the best in business. We know with a large following comes a large
responsibility as we have everyone from institutional investors to the beginner following our profiled
securities in our newsletters. This is something we take very seriously always seeking small cap growth
companies that have both near and long-term potential for our members.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our
VIP SMS/text alert service for free, simply text the word “Alerts” to the phone number 25827 from your
cell phone.
NVTA- Release
Invitae announces major expansion of its test menu for neurological, pediatric, and rare genetic conditions and introduces new panels for inherited metabolic disorders and newborn screening confirmation
Company achieves mid-year goal of more than 1,000 genes in production
Hosting an investor call today at 4:30 p.m. ET
Invitae announces major expansion of its test menu for neurological, pediatric, and rare genetic conditions and introduces new panels for inherited metabolic disorders and newborn screening confirmation
Company achieves mid-year goal of more than 1,000 genes in production
Hosting an investor call today at 4:30 p.m. ET
Invitae Corporation (NYSE: NVTA), a genetic information company, today announced that it has expanded its genetic testing offering with hundreds of additional genes and expanded panels for neurology, pediatrics, and rare diseases. Invitae also has introduced an entirely new clinical testing area designed to complement newborn screening for metabolic and immunological conditions.
With this expanded test menu, Invitae will be able to provide clinicians, patients, and payers with high-quality genetic information for a greatly expanded number of genes and disorders, all at the same price and with the same three-week average turnaround time. The new panels will be available immediately to children’s hospitals, pediatricians, and medical genetics professionals for clinical diagnosis and care.
“We are proud to now offer more comprehensive testing panels for patients who may be suffering from a variety of disorders, including neuromuscular, pediatric, and a host of rare conditions. We’re also now introducing testing for inherited metabolic conditions and immunodeficiencies that are part of most newborn screening programs,” said Randy Scott, chairman and CEO of Invitae. “With this menu expansion, we now have more than 1,000 genes in production a full quarter ahead of our 2016 plan to achieve this goal by mid-year and are on track to continue expanding our test menu while lowering the cost of goods sold in 2016.”
Invitae’s expanded offering includes:
- Expanded neurological menu: Testing for more than 30 disorders including muscular dystrophies, myopathies, as well as congenital myasthenic syndrome (CMS) and malignant hyperthermia susceptibility.
- Expanded pediatric and rare disease menu: Testing for more than 60 disorders, including severe combined immunodeficiency (SCID), periodic fever syndromes, and 20 additional rare diseases.
- New inherited metabolic disorders and newborn screening confirmation: Testing that covers the majority of metabolic diseases for which over 4 million U.S. newborns are screened each year plus a panel covering the common congenital disorders of glycosylation.
Expanded Neuromuscular Panels
Invitae’s neurology offering now includes comprehensive testing for muscular dystrophies, myopathies, and congenital myasthenic syndrome, and tests for conditions such as small fiber neuropathy and malignant hyperthermia susceptibility. Invitae has designed its neurology testing panels to be indication-based, to guide appropriate testing consistent with clinical presentation and diagnostic muscle biopsy.
“As an advocate for people with muscle disease, I see firsthand how important it is to have a genetic diagnosis,” said Sarah Foye, Congenital Muscle Disease International Registry (CMDIR) governing board president. “A comprehensive panel can help families find an answer quickly and potentially guide them to the best care for that condition, leading to improved health and quality of life. A firm genetic diagnosis can also help to predict medical risks associated with that specific condition, and is often required to access a clinical trial working towards a treatment. As a mom and advocate, I know how critical finding an answer is.”
“Our neuromuscular panels are thoughtfully designed and structured for flexible ordering based on clinical presentation and observations from neuropathology, which is unique,” said Tom Winder, PhD, FACMG, medical geneticist at Invitae. “This update to our neurology offering gives clinicians strong, affordable testing options, especially for highly heterogeneous disorders like the muscular dystrophies.”
Pediatric and Rare Disease Panels
Invitae’s pediatric testing menu now covers pediatric immunology, including the most common forms of severe combined immunodeficiency (SCID) and periodic fever syndromes. Invitae has also expanded its rare disease offering to include testing for 20 additional rare diseases, including Batten disease, Carpenter syndrome, and cerebral cavernous malformations, among other conditions.
“The sooner an expanded testing panel is employed in the diagnosis of an acutely ill, undiagnosed child, the greater the value of the test in terms of reducing other diagnostic expenses and speeding beneficial care,” said Steven Bleyl, MD, PhD, medical director, Clinical Genetics Institute at Intermountain Healthcare. “Both lower costs and lab efforts to improve accessibility help me maximize this value for my patients.”
“Pediatric rare diseases can be a roller coaster for families, especially when looking for answers and treatments,” said Allison Moore, founder and CEO, Hereditary Neuropathy Foundation. “Breaking down the barriers to lower costs and increase accessibility will help us move more quickly to an accurate diagnosis of rare pediatric diseases, saving children and their families from extended diagnostic odysseys that waste time and money and take a steep emotional toll on families.”
Invitae has also added disorders of sex determination and dermatologic disorders to its pediatric testing menu, among other additional specific syndromes, in order to broaden its overall pediatric offering.
“Having seen so many parents searching tirelessly for answers for their children suffering from pediatric and rare conditions, my colleagues and I at Invitae are excited to be able to provide comprehensive, high-quality tests at prices that families can afford,” said Robert Nussbaum, MD, chief medical officer of Invitae. “Often a genetic test can provide these parents with definitive answers as to what is happening with their child, and the sooner we can get these answers, the more management options become available to the patient and the family. This can make all the difference to a child’s health and quality of life, and to a parent’s peace of mind.”

Inherited Metabolic Disorders and Newborn Screening Confirmation Panels
Every year, approximately 2% of the estimated 4 million new births in the U.S. result in a genetics related health condition for which genetic testing may be required. These babies are screened for inherited metabolic disorders by analysis of abnormal levels of various substances in newborn blood spots, known as analytes. The initial screens are limited in the number of conditions and genes they analyze and each positive result requires confirmation. Furthermore, approximately 10% of all newborn babies are transferred to a neonatal intensive care unit (NICU) each year, usually because of prematurity. A significant percentage of NICU babies will have abnormal newborn screening results that can be ambiguous because of the infants’ prematurity or the need for intravenous nutrition or other medical conditions. Genetic panel tests, such as those offered on Invitae’s metabolic/newborn screening test menu, provide an efficient, affordable option to help resolve these inconclusive cases.
“Time is of the essence in this field of testing – the longer inborn errors of metabolism go undetected and untreated, the greater the damage. Inherited metabolic disorders are complex, and require thorough answers,” said Olaf Bodamer, MD, PhD, associate chief genetics and genomics at Boston Children’s Hospital. “Newborn screening is one of the greatest public health initiatives; it is, however, a screening test. Confirmatory genetic testing can lead to a diagnosis more quickly and allow for timely initiation of effective therapies, accurate genetic counseling, recurrence risk assessment, and carrier testing for family members.”
Many of these inborn errors of metabolism trigger serious health problems, including nutritional deficiencies, developmental disorders, and premature death. However, if these conditions are caught early, treatments and interventions can avert metabolic crises and their related health problems.
“A family with a baby that is seriously ill needs affordable answers they can trust,” said Nicole Boice, CEO and founder,Global Genes. “Making genetic testing more accessible will extend the power of newborn screening and help impact lives for the better. For babies testing positive for a serious condition, rapid identification and treatment can drastically change the impact of the disease on the child. Such testing can also eliminate lengthy periods of uncertainty during which unnecessary costs are incurred due to misdiagnosis.”
Invitae’s metabolic disorders offering is a strong option for clinicians seeking high-quality, broad, affordable testing for the vast majority of metabolic diseases on the U.S. Recommended Uniform Screening Panel as well as additional inherited metabolic disorders. Invitae offers panels based on disorder and/or analyte results, with flexible panels that clinicians can customize to reflect their own diagnoses and expertise. Invitae is currently the only laboratory to offer “differential diagnosis” panels based on which analyte or analytes are abnormal.
In addition, Invitae’s metabolic testing menu is a strong option for symptomatic patients who need testing after the newborn period, either because their condition is not one of the conditions screened for in the state in which the infant was born, is not detectable on newborn screening, was missed on newborn screening, or because newborn screening was never performed. Invitae’s metabolic disorders testing menu is also appropriate for symptomatic individuals born prior to the availability of expanded newborn screening. Many inherited metabolic conditions only become detectable biochemically during episodes of illness and stress, making DNA-based diagnostics a reliable form of testing even when the individual shows no signs of the disease at the time the test is performed.
“Invitae’s test menu for inherited metabolic disorders puts a significant new testing option in the hands of clinicians caring for families at a time of acute need, and allows us to serve a new group of patients seeking reliable, accessible genetic answers,” said Britt Johnson, PhD, FACMG, molecular and biochemical geneticist at Invitae. “Our panels based on abnormal analyte results allow clinicians to quickly assess the entire spectrum of possible diagnoses and avoid sequential testing – ultimately shortening the time to diagnosis and improving outcomes for patients.”
Invitae Pricing
Invitae offers a transparent pricing structure independent of the number of genes required to provide an accurate diagnosis for any specific clinical indication. For payers and institutions that are in contract with Invitae, the price per indication can be as low as $950, depending on the payer’s requirements. For third-party payers with whom Invitae is out-of-network and for non-contracted institutions, the price per indication is $1,500. In addition, for patients without third-party insurance coverage or who do not meet insurance criteria for coverage, Invitae offers its full test menu for $475 per indication for patients whose clinician orders the testing online and who register online and pay in advance for the testing.
Conference Call Details
Invitae will host a live conference call and webcast today at 4:30 p.m. Eastern / 1:30 p.m. Pacific to discuss the expanded testing menu.
The dial-in numbers for the conference call are (877) 201-0168 for domestic callers and (647) 788-4901 for international callers, and the reservation number for both is 80480572.
The live, listen-only webcast for the conference call may be accessed by visiting the investors section of the company’s website at ir.invitae.com. A replay of the webcast will be available shortly after the conclusion of the call and will be archived on the company’s website.
About Invitae
Invitae Corporation’s (NYSE: NVTA) mission is to bring comprehensive genetic information into mainstream medical practice to improve the quality of healthcare for billions of people. Invitae’s goal is to aggregate most of the world’s genetic tests into a single service with higher quality, faster turnaround time, and lower price than many single-gene and panel tests today. The company currently provides a diagnostic service comprising hundreds of genes for a variety of genetic disorders associated with oncology, cardiology, neurology, pediatrics and other rare disease areas. For more information, visit our website at ir.invitae.com.
Safe Harbor Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to the company’s belief that it can accelerate the adoption of comprehensive genetic information into mainstream medical care and realize its mission; the timing of any new testing service releases and the attributes of any such services; the company’s beliefs regarding the benefits of its pricing program; the company’s belief that its expanded test menu will help clinicians to provide patients and providers with testing that is relevant to diagnoses; the company’s goals to continue to expand its test menu while lowering cost of goods sold; the attributes and benefits of the company’s tests to patients, physicians, and payers; and the indicators of the company’s success and its expected actions with respect to those indicators. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially, and reported results should not be considered as an indication of future performance. These risks and uncertainties include, but are not limited to: the company’s history of losses; the company’s need to scale its infrastructure in advance of demand for its tests and to increase demand for its tests; the company’s ability to develop and commercialize new tests and expand into new markets; the risk that the company may not obtain or maintain sufficient levels of reimbursement for its tests; risks associated with the company’s ability to use rapidly changing genetic data to interpret test results accurately and consistently; the company’s ability to compete; laws and regulations applicable to the company’s business, including potential regulation by the Food and Drug Administration; and the other risks set forth in the company’s filings with the Securities and Exchange Commission, including the risks set forth in the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2015. These forward-looking statements speak only as of the date hereof, and Invitae Corporation disclaims any obligation to update these forward-looking statements.
Source: Invitae Corporation
, a genetic information company, today announced that it has expanded its genetic testing offering with hundreds of additional genes and expanded panels for neurology, pediatrics, and rare diseases. Invitae also has introduced an entirely new clinical testing area designed to complement newborn screening for metabolic and immunological conditions.
With this expanded test menu, Invitae will be able to provide clinicians, patients, and payers with high-quality genetic information for a greatly expanded number of genes and disorders, all at the same price and with the same three-week average turnaround time. The new panels will be available immediately to children’s hospitals, pediatricians, and medical genetics professionals for clinical diagnosis and care.
“We are proud to now offer more comprehensive testing panels for patients who may be suffering from a variety of disorders, including neuromuscular, pediatric, and a host of rare conditions. We’re also now introducing testing for inherited metabolic conditions and immunodeficiencies that are part of most newborn screening programs,” said Randy Scott, chairman and CEO of Invitae. “With this menu expansion, we now have more than 1,000 genes in production a full quarter ahead of our 2016 plan to achieve this goal by mid-year and are on track to continue expanding our test menu while lowering the cost of goods sold in 2016.”
Invitae’s expanded offering includes:
- Expanded neurological menu: Testing for more than 30 disorders including muscular dystrophies, myopathies, as well as congenital myasthenic syndrome (CMS) and malignant hyperthermia susceptibility.
- Expanded pediatric and rare disease menu: Testing for more than 60 disorders, including severe combined immunodeficiency (SCID), periodic fever syndromes, and 20 additional rare diseases.
- New inherited metabolic disorders and newborn screening confirmation: Testing that covers the majority of metabolic diseases for which over 4 million U.S. newborns are screened each year plus a panel covering the common congenital disorders of glycosylation.
Expanded Neuromuscular Panels
Invitae’s neurology offering now includes comprehensive testing for muscular dystrophies, myopathies, and congenital myasthenic syndrome, and tests for conditions such as small fiber neuropathy and malignant hyperthermia susceptibility. Invitae has designed its neurology testing panels to be indication-based, to guide appropriate testing consistent with clinical presentation and diagnostic muscle biopsy.
“As an advocate for people with muscle disease, I see firsthand how important it is to have a genetic diagnosis,” said Sarah Foye, Congenital Muscle Disease International Registry (CMDIR) governing board president. “A comprehensive panel can help families find an answer quickly and potentially guide them to the best care for that condition, leading to improved health and quality of life. A firm genetic diagnosis can also help to predict medical risks associated with that specific condition, and is often required to access a clinical trial working towards a treatment. As a mom and advocate, I know how critical finding an answer is.”
“Our neuromuscular panels are thoughtfully designed and structured for flexible ordering based on clinical presentation and observations from neuropathology, which is unique,” said Tom Winder, PhD, FACMG, medical geneticist at Invitae. “This update to our neurology offering gives clinicians strong, affordable testing options, especially for highly heterogeneous disorders like the muscular dystrophies.”
Pediatric and Rare Disease Panels
Invitae’s pediatric testing menu now covers pediatric immunology, including the most common forms of severe combined immunodeficiency (SCID) and periodic fever syndromes. Invitae has also expanded its rare disease offering to include testing for 20 additional rare diseases, including Batten disease, Carpenter syndrome, and cerebral cavernous malformations, among other conditions.
“The sooner an expanded testing panel is employed in the diagnosis of an acutely ill, undiagnosed child, the greater the value of the test in terms of reducing other diagnostic expenses and speeding beneficial care,” said Steven Bleyl, MD, PhD, medical director, Clinical Genetics Institute at Intermountain Healthcare. “Both lower costs and lab efforts to improve accessibility help me maximize this value for my patients.”
“Pediatric rare diseases can be a roller coaster for families, especially when looking for answers and treatments,” said Allison Moore, founder and CEO, Hereditary Neuropathy Foundation. “Breaking down the barriers to lower costs and increase accessibility will help us move more quickly to an accurate diagnosis of rare pediatric diseases, saving children and their families from extended diagnostic odysseys that waste time and money and take a steep emotional toll on families.”
Invitae has also added disorders of sex determination and dermatologic disorders to its pediatric testing menu, among other additional specific syndromes, in order to broaden its overall pediatric offering.
“Having seen so many parents searching tirelessly for answers for their children suffering from pediatric and rare conditions, my colleagues and I at Invitae are excited to be able to provide comprehensive, high-quality tests at prices that families can afford,” said Robert Nussbaum, MD, chief medical officer of Invitae. “Often a genetic test can provide these parents with definitive answers as to what is happening with their child, and the sooner we can get these answers, the more management options become available to the patient and the family. This can make all the difference to a child’s health and quality of life, and to a parent’s peace of mind.”

Inherited Metabolic Disorders and Newborn Screening Confirmation Panels
Every year, approximately 2% of the estimated 4 million new births in the U.S. result in a genetics related health condition for which genetic testing may be required. These babies are screened for inherited metabolic disorders by analysis of abnormal levels of various substances in newborn blood spots, known as analytes. The initial screens are limited in the number of conditions and genes they analyze and each positive result requires confirmation. Furthermore, approximately 10% of all newborn babies are transferred to a neonatal intensive care unit (NICU) each year, usually because of prematurity. A significant percentage of NICU babies will have abnormal newborn screening results that can be ambiguous because of the infants’ prematurity or the need for intravenous nutrition or other medical conditions. Genetic panel tests, such as those offered on Invitae’s metabolic/newborn screening test menu, provide an efficient, affordable option to help resolve these inconclusive cases.
“Time is of the essence in this field of testing – the longer inborn errors of metabolism go undetected and untreated, the greater the damage. Inherited metabolic disorders are complex, and require thorough answers,” said Olaf Bodamer, MD, PhD, associate chief genetics and genomics at Boston Children’s Hospital. “Newborn screening is one of the greatest public health initiatives; it is, however, a screening test. Confirmatory genetic testing can lead to a diagnosis more quickly and allow for timely initiation of effective therapies, accurate genetic counseling, recurrence risk assessment, and carrier testing for family members.”
Many of these inborn errors of metabolism trigger serious health problems, including nutritional deficiencies, developmental disorders, and premature death. However, if these conditions are caught early, treatments and interventions can avert metabolic crises and their related health problems.
“A family with a baby that is seriously ill needs affordable answers they can trust,” said Nicole Boice, CEO and founder,Global Genes. “Making genetic testing more accessible will extend the power of newborn screening and help impact lives for the better. For babies testing positive for a serious condition, rapid identification and treatment can drastically change the impact of the disease on the child. Such testing can also eliminate lengthy periods of uncertainty during which unnecessary costs are incurred due to misdiagnosis.”
Invitae’s metabolic disorders offering is a strong option for clinicians seeking high-quality, broad, affordable testing for the vast majority of metabolic diseases on the U.S. Recommended Uniform Screening Panel as well as additional inherited metabolic disorders. Invitae offers panels based on disorder and/or analyte results, with flexible panels that clinicians can customize to reflect their own diagnoses and expertise. Invitae is currently the only laboratory to offer “differential diagnosis” panels based on which analyte or analytes are abnormal.
In addition, Invitae’s metabolic testing menu is a strong option for symptomatic patients who need testing after the newborn period, either because their condition is not one of the conditions screened for in the state in which the infant was born, is not detectable on newborn screening, was missed on newborn screening, or because newborn screening was never performed. Invitae’s metabolic disorders testing menu is also appropriate for symptomatic individuals born prior to the availability of expanded newborn screening. Many inherited metabolic conditions only become detectable biochemically during episodes of illness and stress, making DNA-based diagnostics a reliable form of testing even when the individual shows no signs of the disease at the time the test is performed.
“Invitae’s test menu for inherited metabolic disorders puts a significant new testing option in the hands of clinicians caring for families at a time of acute need, and allows us to serve a new group of patients seeking reliable, accessible genetic answers,” said Britt Johnson, PhD, FACMG, molecular and biochemical geneticist at Invitae. “Our panels based on abnormal analyte results allow clinicians to quickly assess the entire spectrum of possible diagnoses and avoid sequential testing – ultimately shortening the time to diagnosis and improving outcomes for patients.”
Invitae Pricing
Invitae offers a transparent pricing structure independent of the number of genes required to provide an accurate diagnosis for any specific clinical indication. For payers and institutions that are in contract with Invitae, the price per indication can be as low as $950, depending on the payer’s requirements. For third-party payers with whom Invitae is out-of-network and for non-contracted institutions, the price per indication is $1,500. In addition, for patients without third-party insurance coverage or who do not meet insurance criteria for coverage, Invitae offers its full test menu for $475 per indication for patients whose clinician orders the testing online and who register online and pay in advance for the testing.
Conference Call Details
Invitae will host a live conference call and webcast today at 4:30 p.m. Eastern / 1:30 p.m. Pacific to discuss the expanded testing menu.
The dial-in numbers for the conference call are (877) 201-0168 for domestic callers and (647) 788-4901 for international callers, and the reservation number for both is 80480572.
The live, listen-only webcast for the conference call may be accessed by visiting the investors section of the company’s website at ir.invitae.com. A replay of the webcast will be available shortly after the conclusion of the call and will be archived on the company’s website.
About Invitae
Invitae Corporation’s (NYSE: NVTA) mission is to bring comprehensive genetic information into mainstream medical practice to improve the quality of healthcare for billions of people. Invitae’s goal is to aggregate most of the world’s genetic tests into a single service with higher quality, faster turnaround time, and lower price than many single-gene and panel tests today. The company currently provides a diagnostic service comprising hundreds of genes for a variety of genetic disorders associated with oncology, cardiology, neurology, pediatrics and other rare disease areas. For more information, visit our website at ir.invitae.com.
Safe Harbor Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to the company’s belief that it can accelerate the adoption of comprehensive genetic information into mainstream medical care and realize its mission; the timing of any new testing service releases and the attributes of any such services; the company’s beliefs regarding the benefits of its pricing program; the company’s belief that its expanded test menu will help clinicians to provide patients and providers with testing that is relevant to diagnoses; the company’s goals to continue to expand its test menu while lowering cost of goods sold; the attributes and benefits of the company’s tests to patients, physicians, and payers; and the indicators of the company’s success and its expected actions with respect to those indicators. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially, and reported results should not be considered as an indication of future performance. These risks and uncertainties include, but are not limited to: the company’s history of losses; the company’s need to scale its infrastructure in advance of demand for its tests and to increase demand for its tests; the company’s ability to develop and commercialize new tests and expand into new markets; the risk that the company may not obtain or maintain sufficient levels of reimbursement for its tests; risks associated with the company’s ability to use rapidly changing genetic data to interpret test results accurately and consistently; the company’s ability to compete; laws and regulations applicable to the company’s business, including potential regulation by the Food and Drug Administration; and the other risks set forth in the company’s filings with the Securities and Exchange Commission, including the risks set forth in the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2015. These forward-looking statements speak only as of the date hereof, and Invitae Corporation disclaims any obligation to update these forward-looking statements.
Source: Invitae Corporation
SCS LLC has not been compensated for this article.
SCS LLC was previously compensated up to twenty thousand dollars each by star media llc for the mention of INVT and XXII however those contracts have expired.